
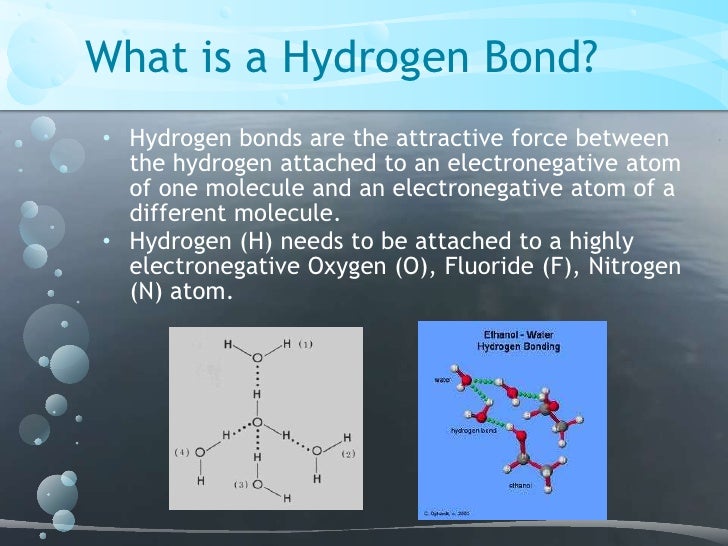
A molecular simulation typically represents less than one millionth of a real sample that is observed during less than one microsecond, and great care must naturally be taken when transferring simulation results to larger scales. However, how simulated microscale properties are related to the macroscale is not always clear. 2020) since they offer both a level of detail that surpasses what can be reached by experimental methods and the ability to quantitatively extract almost any experimental parameter from the simulated ensemble. Molecular modeling such as molecular dynamics (MD) simulations has played an important role over the years for the understanding of molecular-scale phenomena in cellulose (Zhou et al. Interfibril level (how fibrils aggregate into larger structures).įiber and interfiber level (how fibril aggregates are assembled in fibers and how fibers form joints in paper).īased on a critical survey of suitable literature, both old and recent, our aim is to present a more nuanced description of the role of H-bonds in cellulose research.

Interfacial level (how cellulose fibrils interact with other molecules). Intermolecular level (how cellulose molecules interact with each other).įibril level (how cellulose is arranged into crystals). Molecular level (conformation of a cellulose molecule). To this end, we discuss H-bonds in the context of the research on cellulose and their role at different length scales (Fig. Thus, there is a need from time to time to re-examine the claims made with respect to H-bonding in cellulose and cellulose-based materials, and this is the purpose of this review. However, simplistic explanations to complex problems are convenient and thereby tend to survive. Sometimes this explanation is wrong (high modulus and strength of fibrils) or very often incomplete (fiber-fiber bonding and forming of paper), and in most cases the effect of moisture is neglected. For example, unique characteristics of cellulose such as high axial modulus and strength of fibrils, strong fiber-fiber bonding, or forming of paper from fibers are commonly explained based on “hydrogen bonding effects”. But where do all these intriguing and, indeed, extraordinary properties originate from? Although justified in some cases, there is a tendency in the cellulose field to invoke H-bonding as an almost magical explanation. Rapid development in cellulose chemistry, processing and characterization has led to a property range of cellulose-based materials that expanded beyond imagination and to new areas of application that are continuously discovered. Starting with the discovery of nanocellulose and promises of a bright future as sustainable load-bearing component in high performance materials (Berglund and Peijs 2010 Benítez and Walther 2017), the last decade has seen an exponential growth of the interest in cellulose research.

The year 2020 marked the 100-year anniversary of the H-bond concept (Gibb 2020 Pauling 1939), which has, since then, been central for explaining structure-property relationships in biological matter (Jeffrey and Saenger 1994), including cellulose. This mechanism is attributed to the formation of hydrogen bonds (H-bonds) between the nanopaper surfaces (Wang et al. No adhesives are used to seal the roll, but it nevertheless holds together. Consider a wet nanocellulose film that is rolled up and left to air dry, resulting in a nanopaper roll that is sufficiently stiff and wet-stable to be used as a drinking straw.


 0 kommentar(er)
0 kommentar(er)
